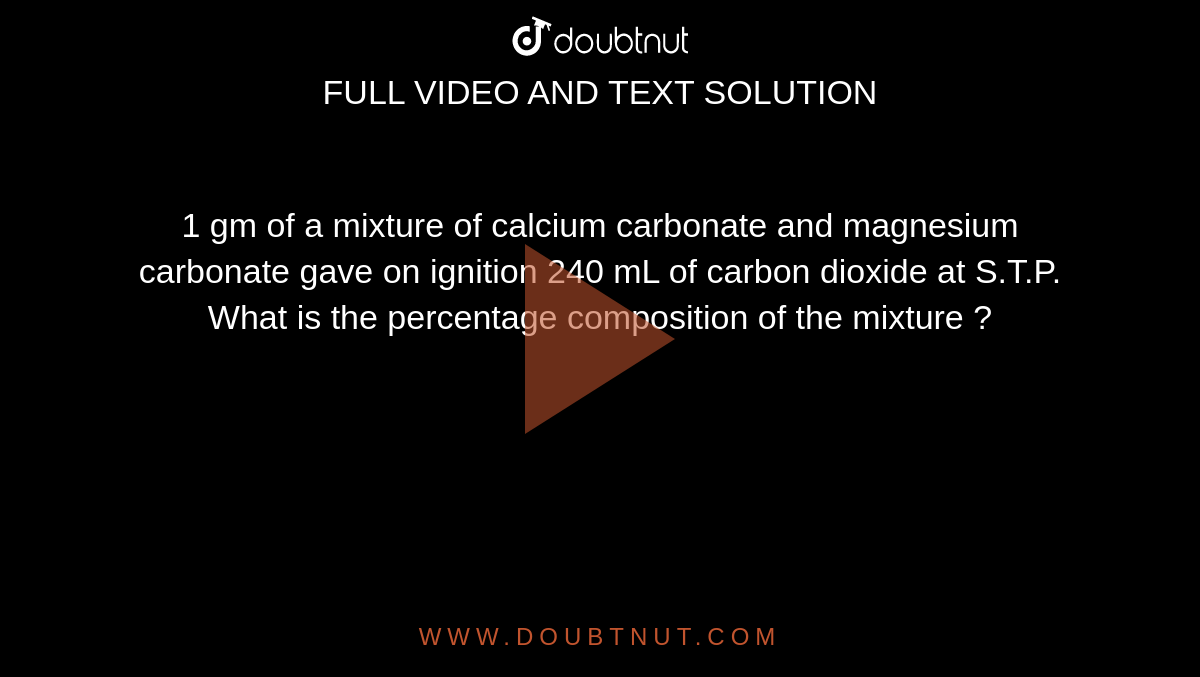
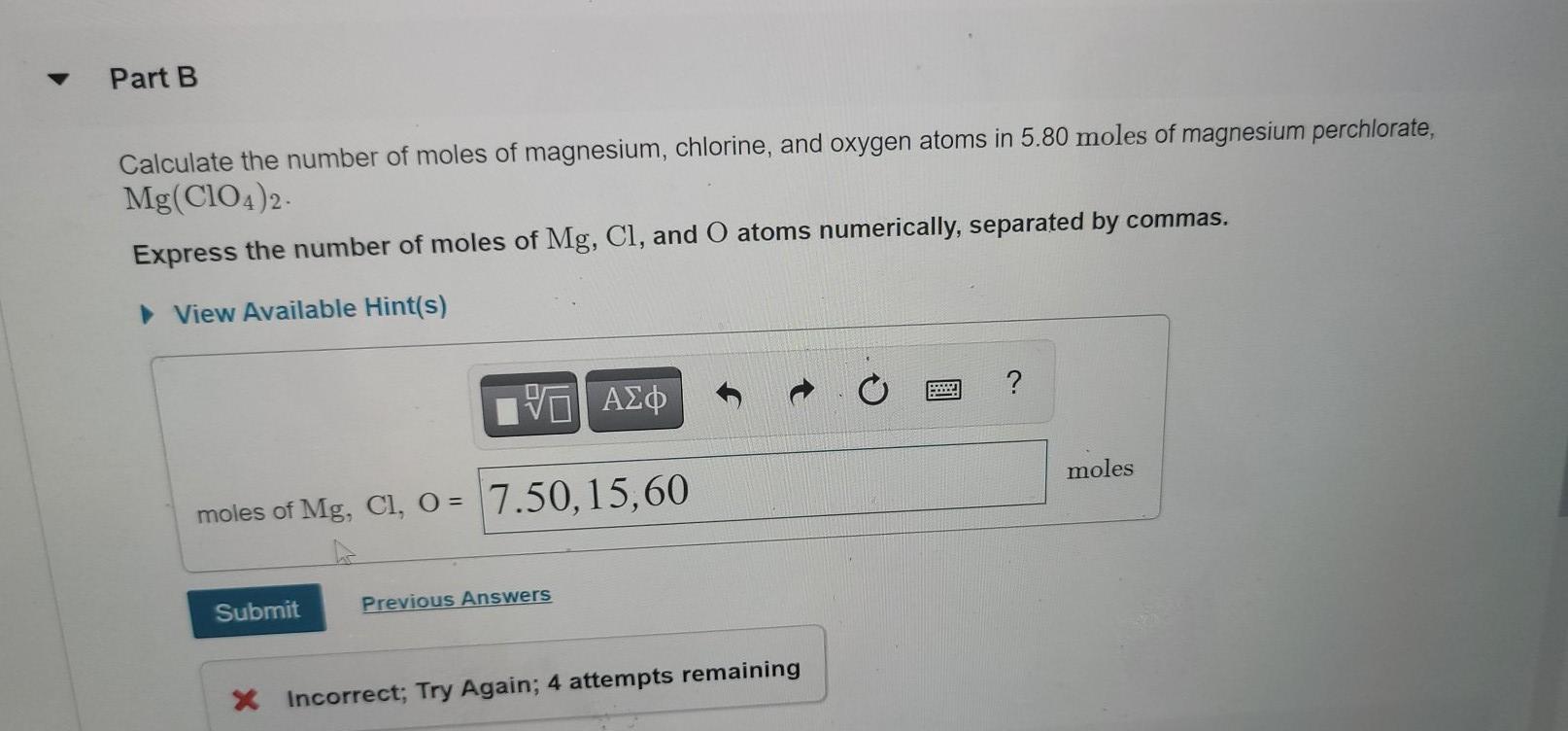
![Calculate the number of molecules present in 0.5 moles of magnesium oxide (MgO) . [Atomic weights : Mg = 24, O = 16 ] Calculate the number of molecules present in 0.5 moles of magnesium oxide (MgO) . [Atomic weights : Mg = 24, O = 16 ]](https://dwes9vv9u0550.cloudfront.net/images/2527137/b4ebf9c0-6b64-43b4-94e9-555f3180cc58.jpg)
Calculate the number of molecules present in 0.5 moles of magnesium oxide (MgO) . [Atomic weights : Mg = 24, O = 16 ]

SOLVED: Calculate the number of magnesium atoms in a 60.0 g sample of forsterite (MgSio4) Be sure your answer has unit symbol if necessary, and round it to 3 significant digits. X1O
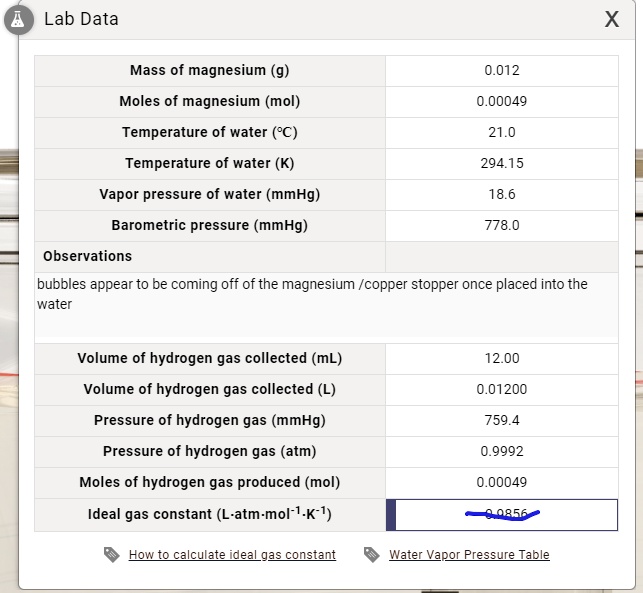
SOLVED: Lab Data X Mass of magnesium (g) 0.012 Moles of magnesium (mol) 0.00049 Temperature of water (PC) 21.0 Temperature of water (K) 294.15 Vapor pressure of water (mmHg) Barometric pressure (mmHg)

SOLVED: 'Magnesium reacts with iron chloride solution. Calculate the mass of iron produced in mg Question Points) Magnesium reacts with iron chloride solution: 3 Mg 2 FeCla 2 Fe 3 MgClz 0.120