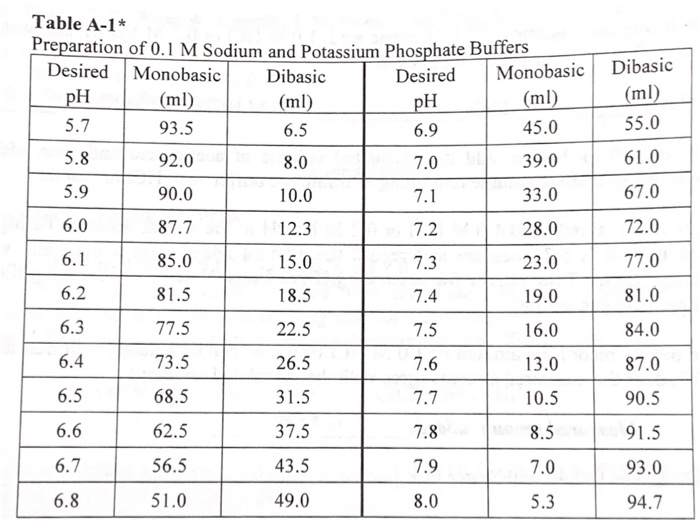
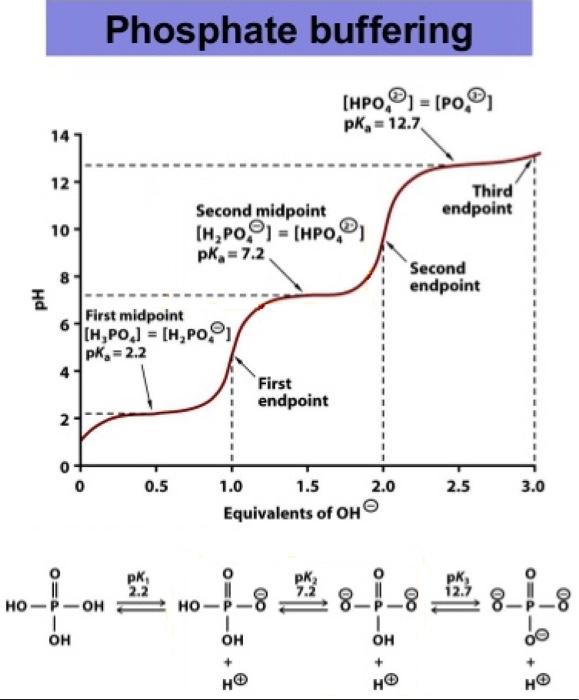
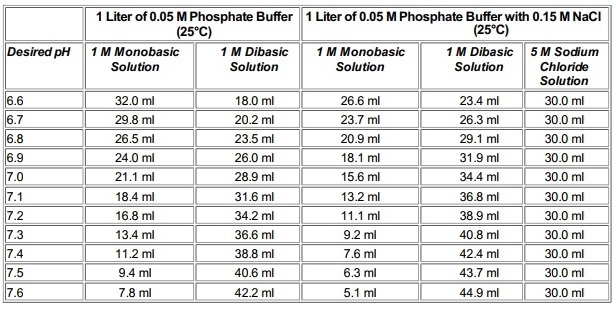
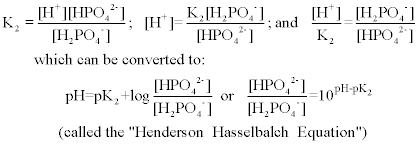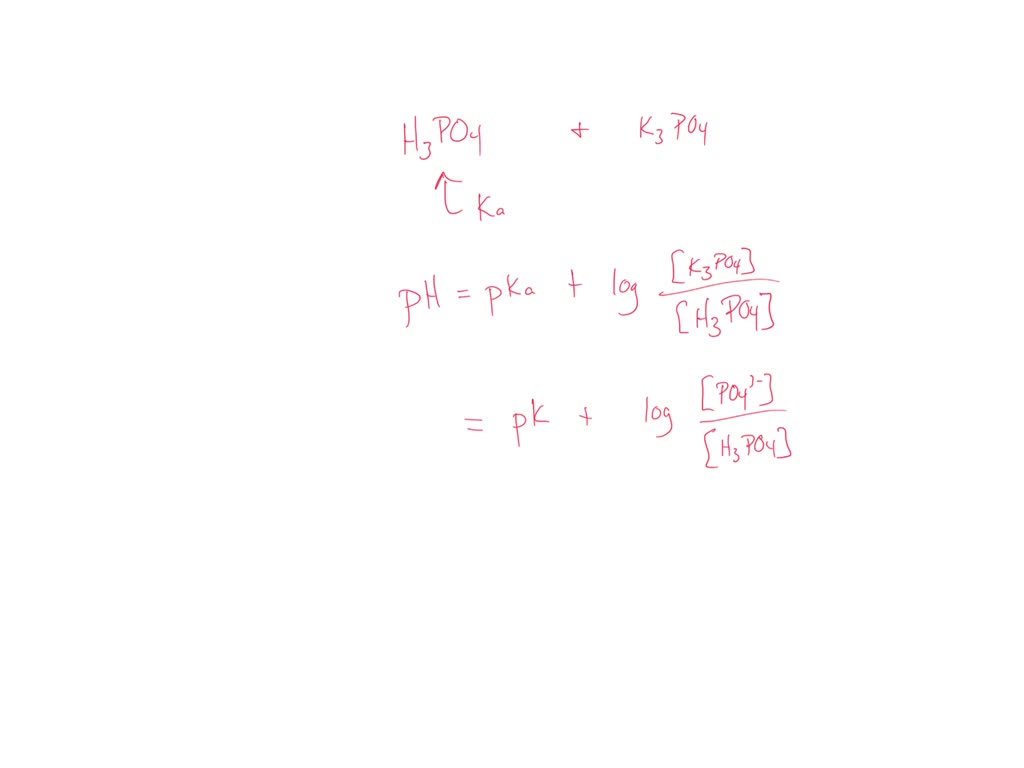SOLVED: You need to prepare 100 mL of a 0.045 M Potassium phosphate buffer ( pH 6.8). Calculate the % base in your buffer. Round your answer to two decimals. Jy moet 100

Concentrations of potassium phosphate buffer and rham- nolipid investigated | Download Scientific Diagram
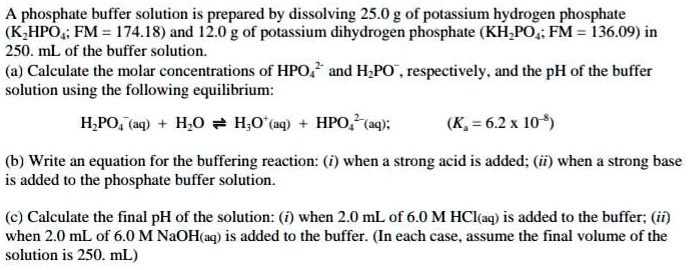
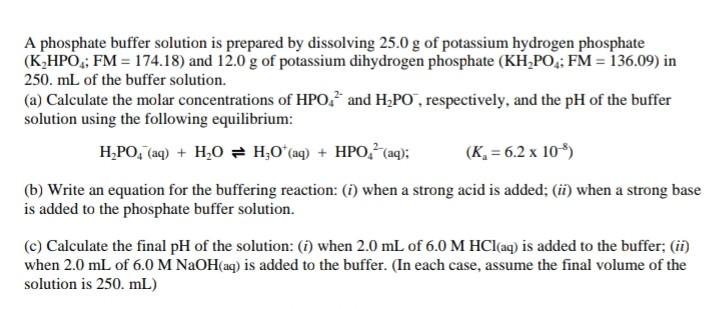
SOLVED: A phosphate buffer solution is prepared by dissolving 25.0 g of potassium hydrogen phosphate (K2HPO4; FM = 174.18) and 12.0 g of potassium dihydrogen phosphate (KH2PO4; FM = 136.09) in 250

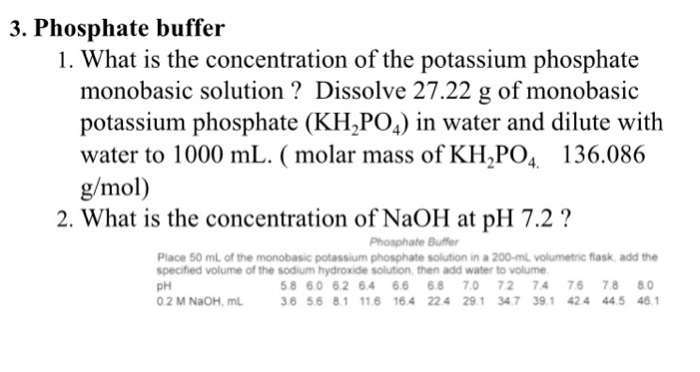
SOLVED: PART B Preparation of a two-component dihydrogen phosphate hydrogen phosphate buffer. You will prepare 250mL of 0.1M potassium phosphate buffer, pH from solid K-HPO4 (potassium hydrogen phosphate) solid KH2PO4 (potassium dihydrogen